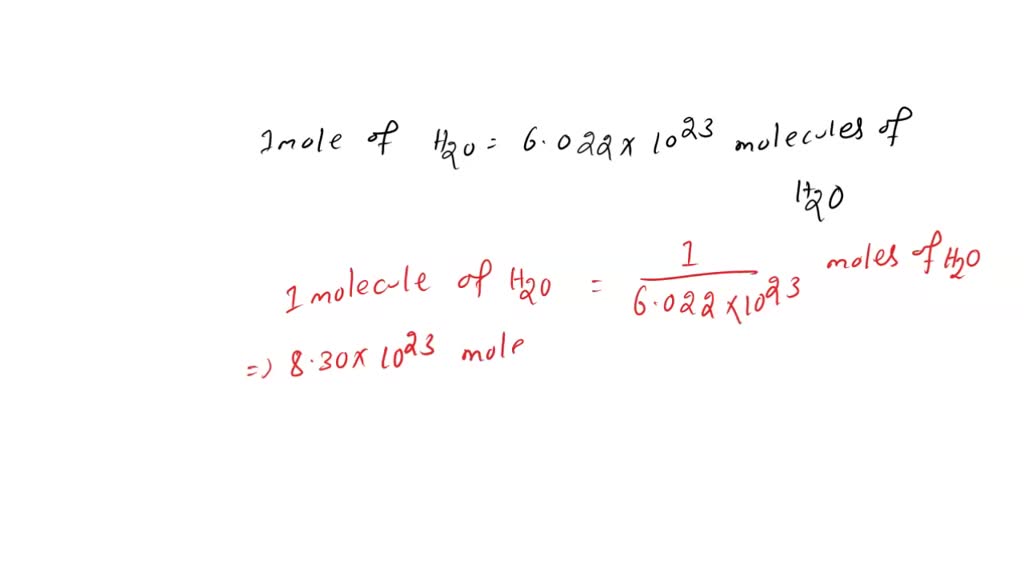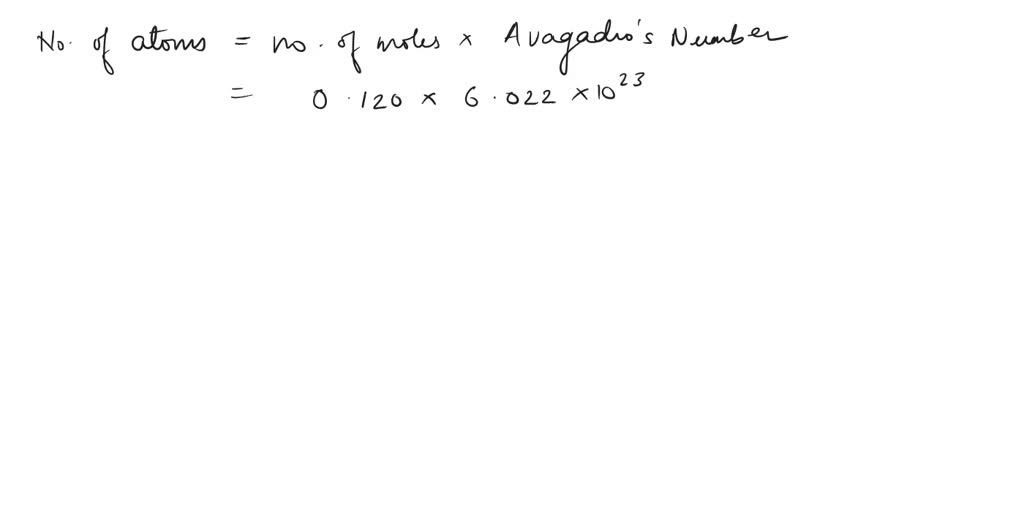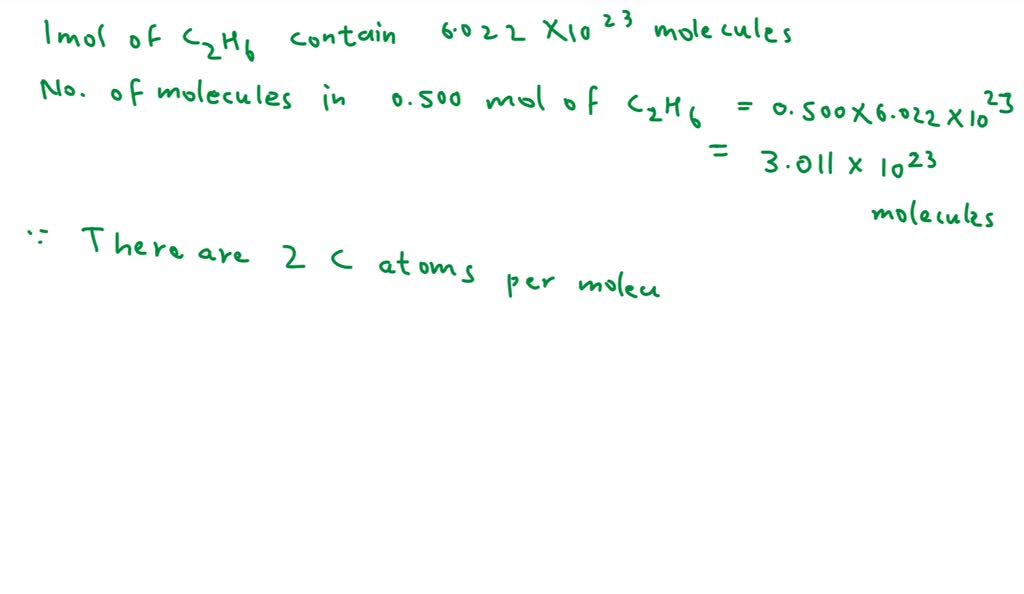Convert 3 01 X 1023 Molecules Of C2H6 To Moles - To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23. Convert 3.01×10 23 molecules of c 2 h 6 to moles. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6. One mole of any substance contains 6.02×10 23 atoms/molecules. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. Use this simple science molecules to moles calculator to calculate mole. Online molecules to moles calculation. 1 mole contains 6.022× 1023 number of molecules. So, there are approximately 0.50 moles of. To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole.
To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. Use this simple science molecules to moles calculator to calculate mole. 1 mole contains 6.022× 1023 number of molecules. Online molecules to moles calculation. One mole of any substance contains 6.02×10 23 atoms/molecules. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. So, there are approximately 0.50 moles of. Convert 3.01×10 23 molecules of c 2 h 6 to moles. To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6.
To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. Use this simple science molecules to moles calculator to calculate mole. Online molecules to moles calculation. So, there are approximately 0.50 moles of. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6. To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23. One mole of any substance contains 6.02×10 23 atoms/molecules. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. 1 mole contains 6.022× 1023 number of molecules. Convert 3.01×10 23 molecules of c 2 h 6 to moles.
SOLVED B How nHY 1 '24 1 moles 8 m 2 molecules water molecules 3 are
Online molecules to moles calculation. So, there are approximately 0.50 moles of. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6. To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. 1 mole contains 6.022× 1023 number of molecules.
Solved How many moles of CO2 are produced when 3.01 x 1023
To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. Convert 3.01×10 23 molecules of c 2 h 6 to moles. Online molecules to moles calculation. One mole of any substance contains 6.02×10 23 atoms/molecules. To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately.
[Solved] How many moles are in 2.58 x 1024molecules of carbon dioxide
To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23. Use this simple science molecules to moles calculator to calculate mole. One mole of any substance contains 6.02×10 23 atoms/molecules. 1 mole.
SOLVED Calculate the number of atoms in 0.120 moles of Na. Calculate
Use this simple science molecules to moles calculator to calculate mole. One mole of any substance contains 6.02×10 23 atoms/molecules. To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23. 1 mole contains 6.022× 1023 number of molecules. Online molecules to moles calculation.
SOLVED Calculate the number of moles of C2H6 (nC2H6) in 6.75 x 10^23
To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. 1 mole contains 6.022× 1023 number of molecules. To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23. Convert 3.01×10.
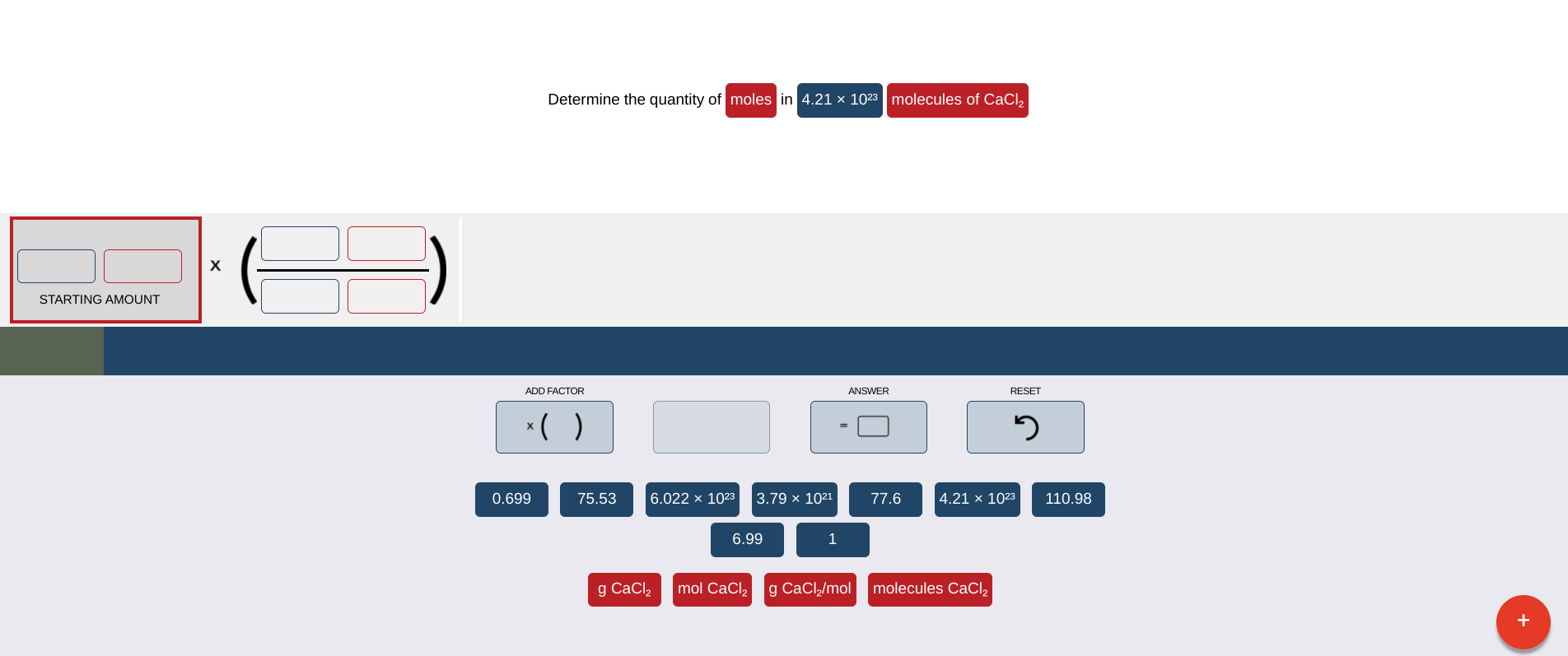
Solved Determine the quantity of moles in 4.21 x 1023
Online molecules to moles calculation. To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. Convert 3.01×10 23 molecules of c 2 h 6 to moles. So, there are approximately 0.50 moles of. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles.
How many moles of carbon atoms are there in 0.500 mole of C2H6? 4.00
To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. Convert 3.01×10 23 molecules of c 2 h 6 to moles. To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per.
Solved How many moles of CO2 are produced when 3.01 x 1023
So, there are approximately 0.50 moles of. Online molecules to moles calculation. Use this simple science molecules to moles calculator to calculate mole. One mole of any substance contains 6.02×10 23 atoms/molecules. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6.
Molecules To Moles
Convert 3.01×10 23 molecules of c 2 h 6 to moles. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. One mole of any substance contains 6.02×10 23 atoms/molecules. To convert molecules to moles, we use avogadro's number, which is approximately 6.022.
SOLVED 1 mol = 6.02 x 1023 particles (atoms or ions or molecules etc
Online molecules to moles calculation. So, there are approximately 0.50 moles of. To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. One mole of any substance contains 6.02×10 23 atoms/molecules. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6.
Online Molecules To Moles Calculation.
(3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. Convert 3.01×10 23 molecules of c 2 h 6 to moles. 1 mole contains 6.022× 1023 number of molecules. To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23.
Calculate The Moles Of C 2H 6 In 3.75× 1023 Molecules Of C 2H 6.
One mole of any substance contains 6.02×10 23 atoms/molecules. Use this simple science molecules to moles calculator to calculate mole. So, there are approximately 0.50 moles of. To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole.